ALZEDETECT- ELISA Based
Based on novel monoclonal antibody (world-wide patent application (PCT) submitted), the kit consists of two complementary ELISAs for reliable (>95% ) diagnostics of early dementia stages. Being recently tested on Shanghai cohort, the kit showed much superior performance compared to other blood biomarkers.
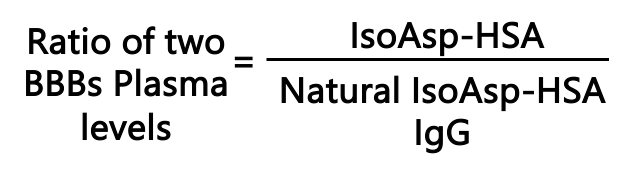
ISOREA’s kit contains two proprietary technologies with complementary blood-based biomarkers (BBBs):
Biomarker-1. Detection of the Risk Factor: By our monoclonal antibody, towards IsoAsp-HSA.
Biomarker-2. Detection of the Natural Protective Antibodies: By a capture protein, that binds to natural blood antibodies for IsoAsp-HSA.

Discovery & Background
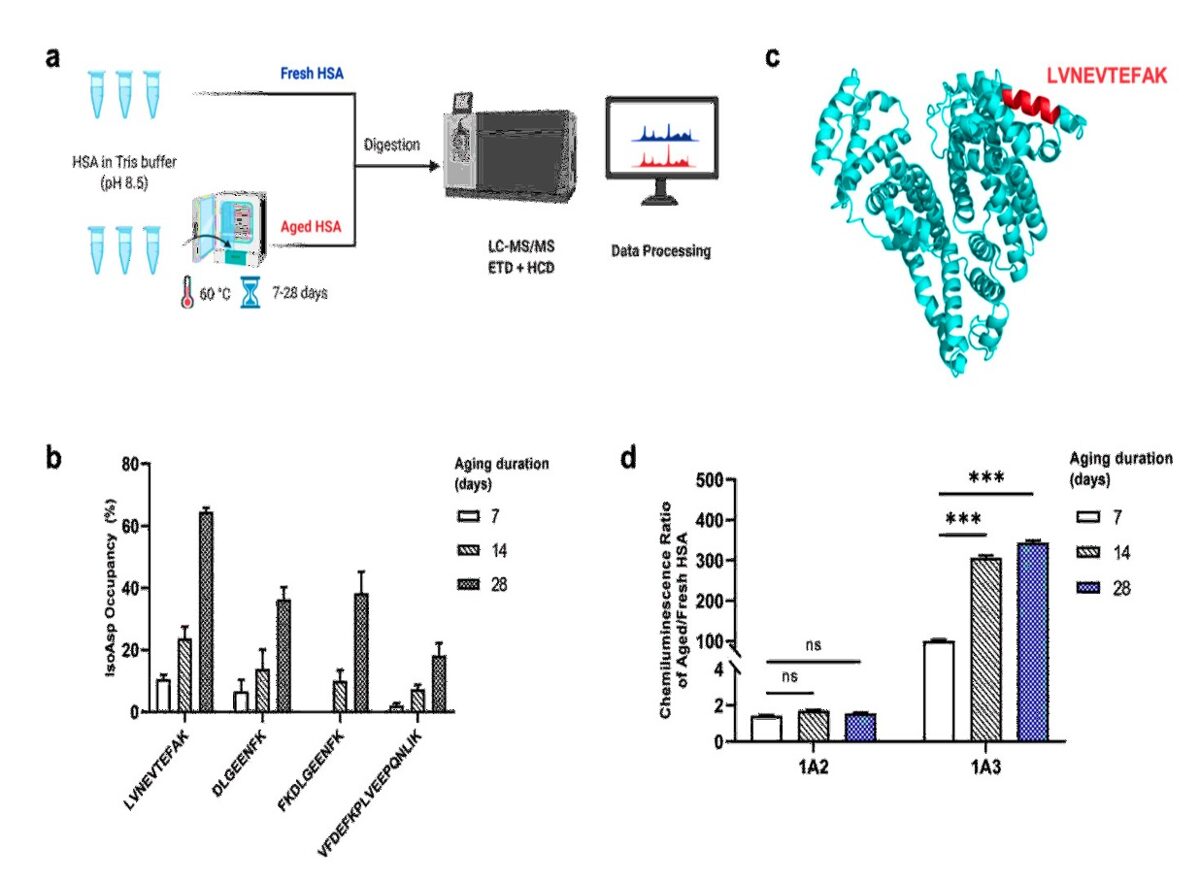
- LC-MS/MS revealed LVNEVTEFAK peptide from a functionally important HSA domain to exhibit the highest deamidation rate in aged HSA.
- A novel monoclonal antibody (mAb) 1A3 generated against LVNEVTEFAK showed excellent specificity to isoAsp in HSA and no cross-reactivity with other aged proteins.
- Using indirect ELISA with chemiluminescence we have measured the range of isoAsp in HSA of healthy blood for the first time, determining the safe region being (0.7±0.3)%.
- We also discovered natural anti-isoAsp antibodies in blood, with their level being reduced in AD. Thus we developed another ELISA using artificially aged HSA as a bait.
- Combination of both ELISA results provides a >95% sensitivity in diagnostics of early dementia.
Find more information in our Publications.